WHEN developing a pipette calibration programme for your laboratory, there are many questions that need to be asked and decisions to be made, write Ann Lenhardt and Erin Lenhardt of Calibrate.
What tolerances should you use? How often should you have your pipettes calibrated? What test points, and how many samples per volume should you request? Do you need As Found (also known as As Received) data? Not every organisation does.
Labs involved in teaching, research and development, or early stage discovery generally do not need As Found data. Neither, for the most part, do labs that are governed by Good Laboratory Practice (GLP) requirements.
Laboratories that do require As Found data include those that comply with Good Manufacturing Practice (GMP) regulations or international standards such as ISO 17025 or 13485 – and those concerned with quality, process tolerances, and traceability. In these cases As Found data is a useful and critical component of pipette calibration.
As Found data is gathered prior to maintenance or calibration. Its purpose is to document the performance of the instrument since its last recorded service event. Each pipette is first identified by serial number and then tested against defined tolerances.
Using ‘as found’ data in pipette calibration
The As Found test should be conducted using at least three samples per volume at two or three different volume settings (typically 10 percent, 50 percent and 100 percent of nominal volume).
A statistical analysis of this data produces the As Found pass/fail status of the instrument. Pipettes that pass this test can be assumed to have been within tolerance since their last documented calibration event, especially when supported by a performance verification programme.
Pipettes that fail their As Found test should be assumed to have been performing outside of tolerance since their last recorded service event.
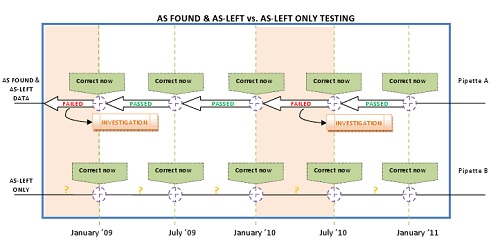
Figure 1 below illustrates the difference between a calibration program that collects and uses both As Found and As Left data, and a programme that collects As Left data only. Both calibration programmes are on a six month interval.
The green flags in the diagram represent the calibration intervals and As Left results for two pipettes, labelled as A and B. While both received preventive maintenance, calibration, and an As Left pass/fail analysis, pipette A also received an As Found performance analysis.
Owners of both pipettes know that their device is within tolerance after the calibration event, and both have the As Left data to review for specific performance statistics. The owner of pipette B, however, knows nothing about the performance of his pipette in between calibration events. His instrument may or may not have been delivering volumes within tolerances between calibration events, or not – there is no way of knowing.
Does your laboratory require pipette calibration traceability?
This type of programme is entirely appropriate for companies whose work does not require traceability. If, however, a company is engaged in work that is governed by state, federal, or international regulations that require traceability and evidence of continually working equipment, an As Left only calibration programme is insufficient.
The owner of pipette A elected to have an As Found performance analysis conducted with each calibration event and has established a chain of traceability for his pipette. Looking at the chart, three out of five As Found tests produced passing results, indicating that his pipette was performing within tolerance between these particular calibration events.
Such evidence also implies that the data produced with Pipette A was within tolerance between these documented service events as well.
The chart above also indicates that pipette A failed its As Found analysis twice in the past two years. The owner must therefore assume there is a good possibility that the instrument was performing out of tolerance at some point since the last calibration event, and the data produced with this pipette may also have been out of tolerance.
There is no way of knowing for how long the instrument has been out of tolerance, and so all data produced with that pipette in that period must be considered suspect.
GMP regulations require that equipment managers ensure adequate and continuous performance of measurement equipment with respect to accuracy and precision. ISO 17025 requires that out of tolerance equipment and any measurements produced with that equipment are investigated to determine their impact.
ISO 13485, which governs the manufacture of medical devices, states: “in addition, the organisation shall assess and record the validity of the previous measuring results when equipment is found not to conform to requirements”.
These standards, and others, require that organisations conduct studies to determine the consequences of having used out of tolerance equipment upon their products, processes, data, and results.
Have a plan ready for pipette failure
It is the view of Calibrate that any lab that requests and documents As Found instrument performance data needs to have a plan in place to address As Found failures.
As Found failures should generate a series of activities (an ‘impact investigation’) to determine the degree to which the instrument was out of tolerance and what impact, if any, that had upon the work.
The goal of the impact investigation is to determine whether or not the pipette could have produced data significantly out of tolerance to the company’s process. All data produced with the pipette between calibration events may be suspect, and of course the longer the interval between calibration events, the more data that must be reviewed and analysed.
Because impact investigations can be expensive and time consuming, many labs also use As Found statistics for performance trending. This enables them to adjust their calibration programme to eliminate As Found failures altogether.
Seeking the root cause of pipette calibration failures
Impact investigations include root cause analyses. When the impact investigation yields a finding of no adverse impact, the root cause of the failure may be related to the calibration programme itself.
Perhaps the tolerances are tighter than necessary, or the calibration interval too long. A calibration programme that includes As Found data provides more than just information about pipette performance, it also provides information about the overall programme and whether this is optimised for effectiveness and economy.
What kind of pipette calibration programme is right? The first step is to determine whether As Found data is a necessary component. Calibrate claims decades of experience assisting customers with these issues to produce the most productive and cost effective pipette calibration programme for their needs.
